- 22 Jul 2024
- 4 Minutes to read
Toxic dispersion
- Updated on 22 Jul 2024
- 4 Minutes to read
Toxic dispersion
Toxic dispersion is the spread of harmful substances into the environment. This occurs mainly through accidental releases from industrial sites or transportation incidents. These substances can harm human health through skin contact, inhalation, or ingestion, and also affect other organisms, leading to environmental pollution at low concentrations.
Exposure to toxic substances can result in various health issues such as respiratory problems, neurological disorders, cancer, reproductive issues, or even death. Understanding toxic dispersion is crucial for minimising these impacts and safeguarding both the environment and public health. The severity of harm from toxic substances depends on factors like the concentration of the substance, the duration and type of exposure, and the availability of protective layers.
For a visual demonstration, the video below depicts the dispersion of chlorine in Trial #7 of the Jack Rabbit II chlorine release tests, where 10 tons of chlorine were released from a tank at a 135-degree angle into a free field (UVU, 2016).
Chemical toxicity
Toxic substances are those capable of causing poisoning or adverse health effects. Assessing chemical toxicity is crucial for workplace safety, where even low concentrations of hazardous substances can be harmful. Threshold values are set to gauge vulnerability to inhaled toxins, guiding safety measures and emergency responses.
In workplaces, Threshold Limit Values (TLVs) and Permissible Exposure Limits (PELs) are key for managing chemical exposure. TLVs, set by ACGIH, and PELs, regulated by agencies like OSHA, help determine necessary protective measures.
In emergencies, various threshold values assess health risks from toxic substances. Immediately Dangerous to Life and Health (IDLH) values signify severe exposure risks. Acute Exposure Guideline Levels (AEGLs), Emergency Response Planning Guidelines (ERPGs), and Temporary Emergency Exposure Limits (TEELs) provide guidance for assessing rare airborne chemical exposures (Bosch, 2005).
Factors influencing toxic dispersion
The guidelines mentioned above establish thresholds for identifying when concentrations of chemicals become hazardous. However, it is equally important to pinpoint where and when exposure to these chemicals poses a risk. Toxic dispersion modelling plays a critical role in forecasting the concentration levels of chemicals in the air at specific locations over time.
The table below provides an overview of the factors that influence how toxic chemicals disperse. By analysing these factors, stakeholders can improve their ability to predict, monitor, and control the spread of toxic chemicals. Ultimately, this enhances environmental safety and protects public health (Bosch, 2005).
Factors | Description |
|---|---|
Wind Speed and Direction | Higher wind speeds typically lead to quicker dispersion and reduced concentrations of pollutants in the air. However, faster winds can also carry the toxic cloud over a greater distance. Furthermore, the direction of the wind determines the path that the chemicals will follow, influencing which areas will be impacted by the pollutants. |
Atmospheric Stability | In stable atmospheric conditions, pollutants may accumulate near the ground, posing a greater risk to human health. Conversely, unstable conditions promote dispersion, leading to a more widespread distribution of pollutants. |
Terrain Features | Features such as mountains, valleys, and urban canyons can alter airflow, creating barriers or channels that influence dispersion patterns. This can result in localised areas of high concentration, particularly in valleys with dense populations that may be at greater risk of exposure. |
Surface Roughness | Rough surfaces can create friction, slowing down the dispersion of chemicals, while smoother surfaces allow for more rapid dispersion. |
Temperature Inversions | Temperature inversions occur when a layer of warm air traps cooler air near the ground, inhibiting the vertical mixing of pollutants. This can lead to elevated concentrations of toxic chemicals at ground levels, posing a heightened risk to nearby populations. |
Assessing toxic dispersion
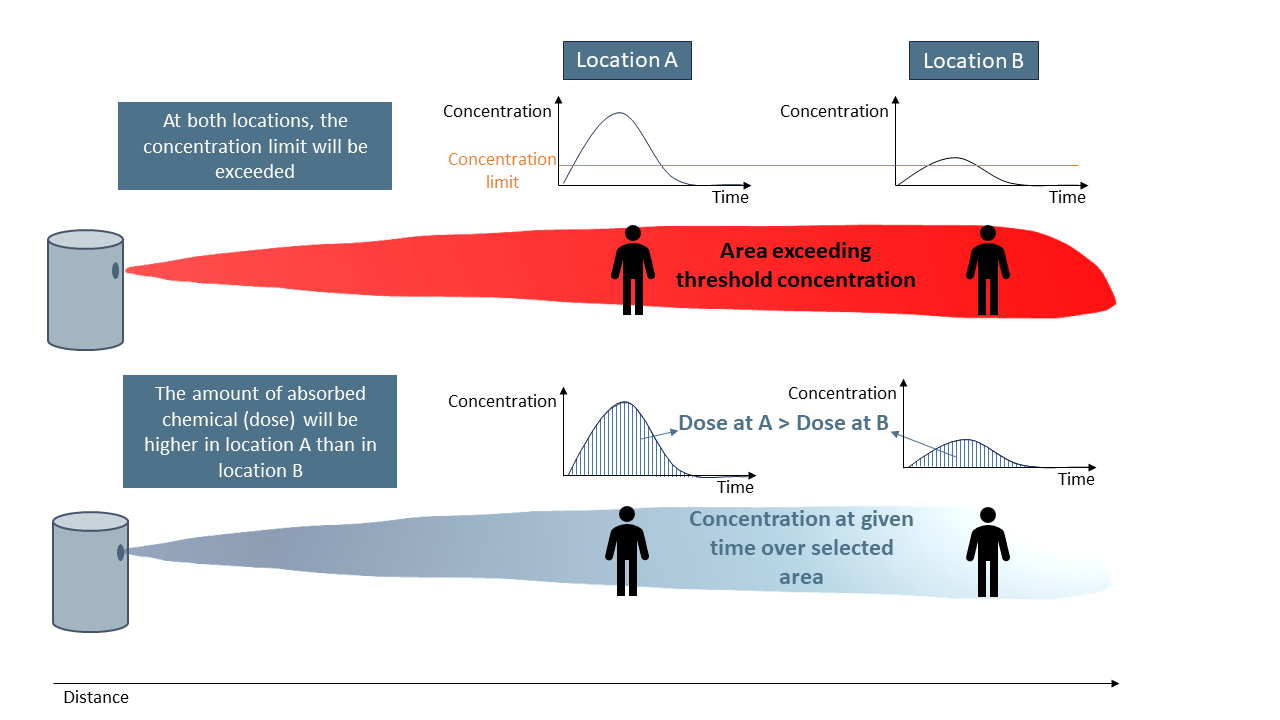
To assess the harmful effects of a chemical released in an accident, it is essential to accurately monitor its concentration in the air at specific locations and over time. Understanding toxic concentration is crucial because the effects of toxins depend not only on their concentration but also on the duration of exposure.
Toxic concentration indicates the presence of a substance at a particular spot. On the other hand, toxic dose, which is calculated by multiplying the concentration by exposure time, provides a comprehensive view of the cumulative impact of chemical exposure. This approach considers both how much of the chemical is present and for how long individuals are exposed. Additionally incorporates the toxicity of the chemical.
It's important to differentiate between toxic dose and toxic concentration. Simply reaching a certain concentration briefly does not necessarily mean that people in that area have absorbed enough of the chemical to experience harmful effects. Therefore, symptoms observed in one population at a specific location may not occur in another population exposed to a similar concentration elsewhere.
Understanding these differences is critical for accurately assessing the risks associated with chemical exposure and implementing effective safety measures (Bosch, 2005).
Conclusion
Understanding and managing toxic dispersion is vital for protecting public health and the environment. Accidental releases of toxic substances can cause severe health problems and environmental damage, making effective monitoring and mitigation essential. Key factors such as wind speed and direction, atmospheric stability, terrain features, surface roughness, and temperature inversions must be analysed to predict and control the spread of hazardous materials.
Chemical toxicity assessments, including Threshold Limit Values (TLVs) and Permissible Exposure Limits (PELs), provide guidelines for workplace safety and emergency response. Modelling and monitoring toxic dispersion helps identify high-risk areas and implement protective measures.
By distinguishing between toxic dose and toxic concentration, we can more accurately evaluate the risks of chemical exposure and develop effective safety protocols. A comprehensive approach to managing toxic dispersion enhances safety standards and promotes public health and environmental sustainability.
References
UVU Emergency Services (2016) JACK RABBIT II Phase II 2016 TRIAL 7: View Looking SouthWest @ 80 Meters. The Jack Rabbit Program. Available at: https://www.uvu.edu/es/jack-rabbit/
Bosch, C. v. (2005). Methods for the calculation of physical effects 'Yellow book' CPR 14E. The Hague: Ministerie van Verkeer en Waterstaat

