- 02 Aug 2024
- 2 Minutes to read
Pool fire
- Updated on 02 Aug 2024
- 2 Minutes to read
What is a Pool Fire?
A Pool Fire can be defined as a turbulent diffusion fire burning above a horizontal pool vaporising flammable material under conditions where the flammable material has zero or very low initial momentum (Cowley, 1991). The footage below from Gexcon’s Fire and Explosion Testing department shows what a Pool Fire looks like.
How does a Pool Fire arise?
Pool Fires occur upon the direct ignition of a flammable liquid spill. Such liquid spill can occur due to the catastrophic rupture of a vessel or accidental leakage from a vessel or a pipe.
There is a degree of feedback between the fire and the flammable material. To a greater or lesser degree, the heat is transferred from the fire back into the pool, which affects or even controls the evaporation rate and thereby, also controls the size of the fire and other burning characteristics.
The wind has a significant influence on the flame shape and burning rate. Pool fires cause danger to the surroundings resulting from their thermal effect, especially from the radiant heat that is released from the flames. To be able to calculate the heat effect on surroundings, parameters such as the pool diameter, burning rate, flame dimensions, surface emissive power, atmospheric absorption, and heat flux need to be assessed.
Characteristics of a Pool Fire
The flame of a Pool Fire can usually be separated into two areas:
Strongly emissive clear flames at the base, which are characterised by a maximum surface emissive power.
Dark sooty (obscured) flames at the top, which are characterised by a reduced surface emissive power (as the soot blocks the amount of heat received).
The division of the clear and sooty flames along with the flame dimension is described in the picture below.
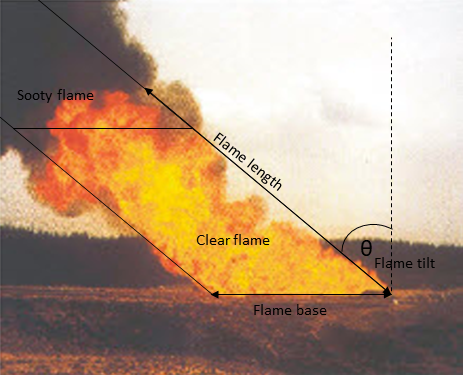
The generation of soot occurs upon the incomplete combustion process of hydrocarbons. As opposed to Jet Fires, Pool Fires more often have a top-sooty flame. That is because Pool Fires occur due to the direct ignition of a flammable liquid (heavy) hydrocarbon (e.g., gasoline, naphtha, etc.). Liquid hydrocarbons have more carbon atoms in their structure, therefore, they are more likely to contribute to the generation of more soot (due to an incomplete combustion process) (Bosch, 2005).
On the other hand, Jet Fires, occur upon the direct ignition of short chains of hydrocarbons (e.g., methane, propane, etc.). Therefore, less soot or no soot will be generated. Additionally, we assume a better mixing with air at the boundaries of a jet flame. Thus, there will be enough oxygen for the combustion process to be complete.
Surface for pool spreading
The surface on which the pool is spreading plays an important role in estimating the base of the flame. Pool Fires can be present on land or water. A typical instance of a Pool Fire on land involves a tank pit fire fueled by heavy hydrocarbons or a leak from a vessel transporting LPG (liquefied petroleum gas) or LNG (liquefied natural gas). Conversely, Pool Fires on water are commonly associated with the maritime transportation of flammable materials, including LNG, LPG, crude oil, and fuel oils by ships. In the case of some substances, such as ammonia, the substance not only burns but dilutes with water at the same time. Predicting the latter phenomenon still presents a challenge for current technologies.
.png)
Reference
Cowley, L.T. and Johnson, A. D. (1992). Oil and gas fires: characteristics and impact. Great Britain. Health and Safety Executive Steel Construction Institute.
Bosch, C. v. (2005). Methods for the calculation of physical effects 'Yellow book' CPR 14E. The Hague: Ministerie van Verkeer en Waterstaat.

