- 02 Aug 2024
- 9 Minutes to read
Models and parameters
- Updated on 02 Aug 2024
- 9 Minutes to read
General
Can I simulate scenarios with solids in EFFECTS?
EFFECTS includes two models available for solids.
A fire model ‘‘(Toxic) Combustion Products’’ which is typically used to calculate the formation rate of toxic combustion products when a warehouse fire scenario occurs.
An explosion model ‘‘Solid Explosion’’, which is often referred to as the ‘‘TNT model’’. This model can be used to calculate overpressure and overpressure damage resulting from solid explosions based on mass or energy.
All other models are focused on gases, liquids or liquefied gases.
What CO2 (carbon dioxide) scenarios can I simulate in EFFECTS?
EFFECTS models support CO2 releases in several ways:
The "Statistical Spray Release" model implemented into our release models accounts for:
Solid-vapour equilibrium of CO2, as the triple point of the substance lays above atmospheric pressure.
Flashing generating solid CO2 particles (snow).
Rain-out leads to the generation of a solid layer.
The "Dispersion" model takes into account the vaporisation of snow particles airborne contributing to the generation of a bigger cloud.
The "Liquefied gas from long pipeline’’ model is validated for CO2 release for both full-bore and non-full bore ruptures (hole size >20% of inner surface area).
Do models account for solubility in water? How to deal with water-diluted mixtures?
Water-diluted mixtures cannot be dealt with directly in EFFECTS because the two constituents of the solution usually have a polar behaviour. If you create a mixture containing water and for instance, nitric acid or ammonia, EFFECTS will assume “ideal mixing” (based on Raoult’s law) using partial vapour pressure and not take into account how this mixture will have its properties affected by binary interactions or solubility. As a result of neglected properties (i.e., binary interactions and solubility), the real mixture behaviour will not be reflected in the simulations which is highly relevant for the calculate on of the pool evaporation phenomenon. The main challenge is that the curve “vapour pressure vs temperature” of your newly created mixture will not be completely accurate. Thus, the normal boiling point and its range will also not be completely accurate.
Solution:
If the liquid vapour pressure deviates from the ideal mixture, you can follow the steps in the technical note “Technical Note. Thermodynamics. Modelling Water Diluted Mixtures.pdf’’ so that water-diluted mixtures can be dealt with in EFFECTS. This document is available on the Customer Support & Downloads Centre. You can read more about the limitations of the chemical database in the article Chemical database in EFFECTS and RISKCURVES.
What are the limitations of the software?
Mixtures:
No binary interaction, No chemical reactions, No absorption effects, No solubility
We assume constant composition (also within pool evaporation):
The chemical for dispersion has the same composition as a liquid in the pool (when linked)
Based on quadratic EOS including compressibility based on the second virial coefficient (supercritical conditions are less accurate)
Dispersion:
The influence of large obstacles on concentrations can be big (not included). Short-scale modelling requires detailed 3D definition surroundings. This is the typical field of application for Gexcon’s CFD software: FLACS-CFD.
The atmosphere is stochastic in nature but meteorological conditions are dynamic in reality. Wind speed, direction and stability will vary with location, height and time: Dispersion models are applicable to a maximum of 10 km.
Explosion:
The EFFECTS Receivers use a simplified approach to model the effects of explosions in congested areas and cannot fully capture the complex interactions and dynamics present in complex 3D congestion areas. This is the typical field of application for Gexcon’s CFD software: FLACS-CFD.
How do I estimate the size of a pool?
The pool may be limited by the bunds or it may spread. The Pool Evaporation model may be used to calculate the diameter and surface area of a spreading pool that can be connected to your Pool Fire model.
However, the Pool Fire model does not necessarily require the size of the pool. Instead, a drawing of the shape of a pool can be used, which will determine the necessary dimensions and will inform you about the size of the pool. Explore how to drawn any pool shapes in EFFECTS in the article How to simulate tank pit fires.
Where can I find the theoretical background of an EFFECTS model?
EFFECTS models are based on the Colour Books which can be downloaded from Gexcon’s webpage. These coloured books were published in the 80s due to research conducted by the Dutch government and in collaboration with TNO (the Dutch Research Institute for Applied Scientific Research). The coloured books are no longer updated, which is the reason why EFFECTS & RISKCURVES are still improved and developed based on the most recent research, and other literature sources such as the CCPS guideline books, HSE publications or local regulations.
The latest model background literature, technical notes and validation reports are available for all EFFECTS users on the Customer Support & Downloads Centre.
What exposure times to use?
A default toxic exposure duration value of 30 minutes (1800 seconds) is used by the model because it is expected that a person will seek a shelter withing this time.
A heat exposure duration is set to a default of 20 seconds because the human reaction to intensive heat radiation is much quicker.
These values can be changed in the Vulnerability section of the model input.
Fire and explosion
Why is the Surface Emissive Power (SEP) of hydrogen limited to 70 kW/m2?
When dealing with pure hydrogen, the heat radiation from the flame surface is very low (in the range of 70 kW/m2; which corresponds to an almost invisible flame radiating in the far infrared). To properly model the hydrogen jet fire, the jet fire model will force the surface emissive power (SEP) to be ≈70 kW/m2, leading to more realistic radiation contours.
The value is based on experiments during the SH2IFT project and is also suggested in the experimental data presented by Rew & Hulbert. The limit is applied to avoid overly conservative results. Be aware that methane/hydrogen mixtures do have a much higher SEP (>>70 kW/m2).
Can EFFECTS model Dust Explosions?
Unfortunately, EFFECTS does not support dust explosions or dust dispersions, which would need a solid deposition model. The blast force of a dust explosion event highly relates to the material strength of the enclosure (steel, concrete) which requires a highly detailed material/geometry definition, while the associated energy relates to the type of material, which could be starch, wood dust, milk powder, etc. These are not the kind of materials you can find in the DIPPR database. Therefore, dust explosions are not supported within EFFECTS or RISKCURVES.
Note that Gexcon FLACS-CFD, which is a CFD software tool aimed at accounting how the flow interacts with complex 3D surrounding geometries can be used to model dust explosions. "
Furthermore, there are several "rules of thumb" models which you might apply. These models are publicly available from external sources. The overpressure results from such an approach can be entered as an explosion consequence footprint in RISKCURVES.
Can I simulate explosions using a TNT equivalence factor in EFFECTS?
Yes, the ‘‘Solid Explosion’’ model can be used for this purpose. The “Solid Explosion” model is often referred as the ‘‘TNT model’’, which can be used to calculate overpressure and overpressure damage resulting from solid explosions based on mass or energy. When using mass mass-based approach, one would simply define the equivalent amount of TNT mass.
Can EFFECTS model a Jet fire from buried pipelines?
This model is not yet supported, but a release model for buried pipelines will become available in 2024.
Dispersion
What is the difference between the Neutral Gas Dispersion and Dispersion models?
Dispersion model:
A new model available since EFFECTS and RISKCURVES v12.0 were released.
The mathematical approach is based on the description of the entire physics of the dispersion phenomenon and accounts for density differences, making it suitable for dense, neutral and lighter-than-air gas dispersion. This improved dispersion model also considers transition behaviour (e.g., from dense to light which is the case for refrigerated ammonia releases).
Neutral Gas model:
A previous model was kept as an ad-hoc model.
Only for “pure passive” situations (no influence impulse, density differences, droplets).
Allows to define a release window of continuous releases, for instance, a warehouse fire.
What is the difference between Concentration, Toxic dose and Flammable Dispersion models?
Concentration: User defined concentrations can be tracked over at time and distance.
Toxic dose: Tracks cumulated (inhaled) toxic dose over distance based on toxic dose results (Toxic dose = Cn ∙ t) .
Flammable cloud: Based on flammable concentration (LFL). Thacks explosive mass in the cloud (above LEL) over time and distance.
How to set Probit for suffocation by inert gases?
Even inert gasses can cause suffocation when released in large quantities because they simply can displace the oxygen. In general, the hazards associated with the release of inert gases are negligible. Only in case of very large release rates or large quantities of inert gas released in enclosed environments, such as refrigerated storage facilities for producers, it might be relevant to evaluate the risk of release of inert substances. In that case, the gas being released (e.g., Nitrogen) should have Probits defined according to the relation below:
Pr = -65.7 + ln (∫ C5.2 dt)
Where:
probit A = -65.7 (units ppm*min),
probit B = 1
probit N = 5.2 (RIVM, 2018)
For any chemical that the user wants to evaluate on potential suffocation as inert gas, these Probits will need to be entered:
Create a user-defined chemical based on the original DIPPR substance (we advise to immediately adjust the name according to its purpose, e.g. “NITROGEN Suffocating Inert Gas”)
Add a Probit A, Probit B and Probit N. Note that the probits MUST be entered in SI units. Adjust the A probit value in [kg/m3.s] as calculated with the probit converter. Then enter value 1 for B probit and 5.2 for N probit. The probit value expressed in SI = [kg/m3.s] units can be recalculated by applying the Probit converter toll in the EFFECTS tools menu. Note that this conversion requires the molar mass of the chemical. The molar mass can be directly found in the database under constant properties. This molar weight should be added when converting probits to SI units.
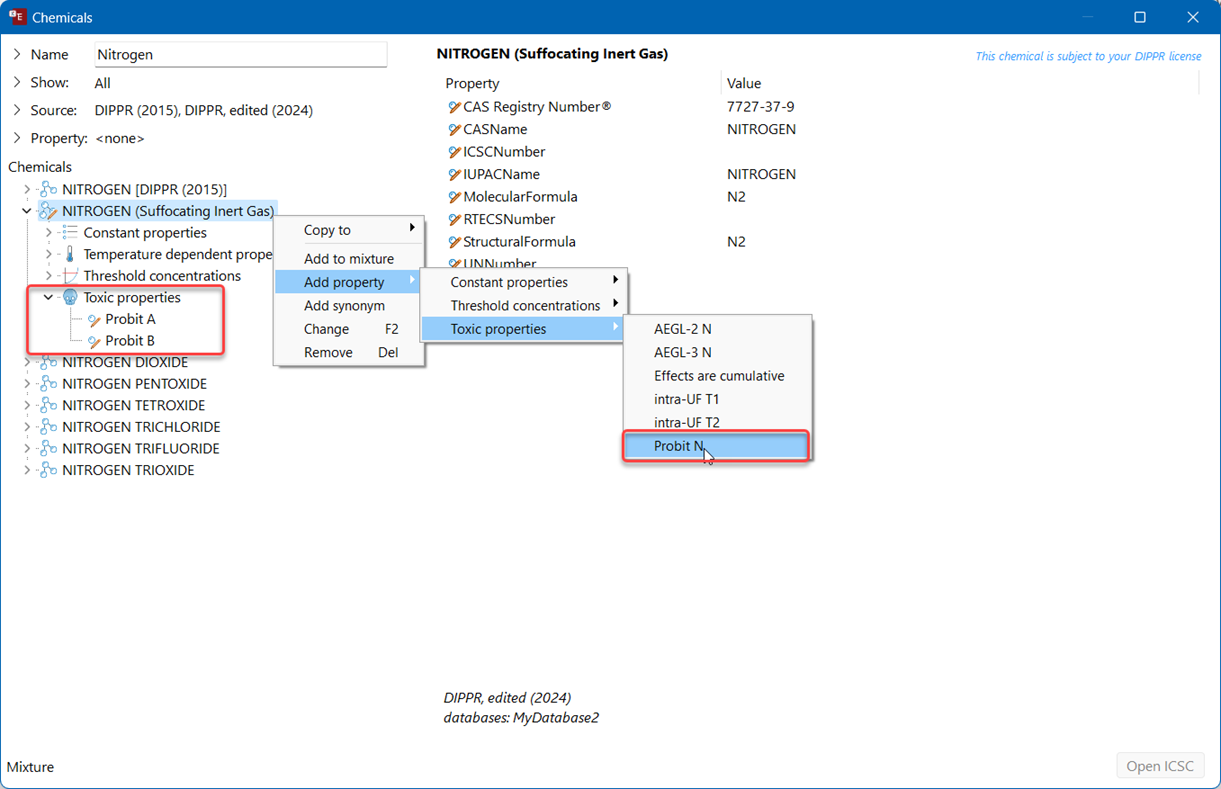
Validate whether all 3 values for A, B and N have been accepted and are reproduced. When selecting the [ppm.min] unit for the A probit, the number -65.7 should appear:
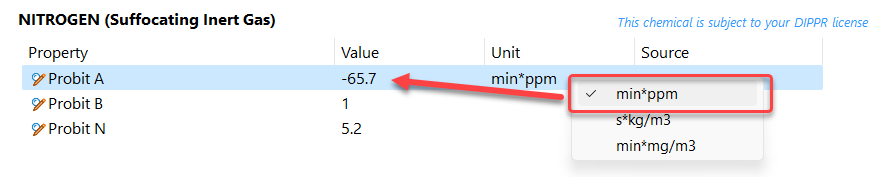
This Chemical can now be used in a “Dispersion – Toxic dose” model to calculate the risk of suffocation!
Reference:
RIVM (2018). Reference Manual Bevi Risk Assessments Version 3.2. National Institute of Public Health and the Environment (RIVM). Available online: https://www.rivm.nl/sites/default/files/2018-11/Reference-Manual-Bevi-Risk-Assessments-version-3-2.pdf
How to account for Oxygen risk?
In principle, increased oxygen levels may create a potential fire hazard, because high concentrations will lead to an increased probability of fire in the surrounding area. Usually, the risks associated with the storage of oxygen are neglected, but again, in case of large quantities being stored, it might be sensible to evaluate the enhanced flammability of surrounding materials. In those “large quantity” cases we would strongly advise simply looking at the concentration levels that can be reached in the near surroundings. Especially if the surroundings contain flammable materials as small particles: wood-dust, plastics- and aluminium powders which may burn rapidly when exposed to high oxygen levels, due to the large contact area with the surrounding air.
In those cases, the following oxygen levels can be associated with different lethality levels:
Plethal = 1………………………………Concentration of oxygen higher than 40 vol%
Plethal = 0.01…………………………Concentrations of oxygen between 30 and 40 vol%
Plethal = 0………………………………Concentrations of oxygen between 20 and 30 vol% (RIVM, 2018)
Note that a threshold concentration of:
40 vol% corresponds with an additional quantity of 24.1 vol% of oxygen as calculated by the dispersion model
30 vol% corresponds to a result of 11.4 % from the dispersion calculation.
This is because the dispersion model does not account for the amount of oxygen (21.4 vol%) which is already present in the air. The correlation is based on BEVI guidelines.
Reference:
RIVM (2018). Reference Manual Bevi Risk Assessments Version 3.2. National Institute of Public Health and the Environment (RIVM). Available online: https://www.rivm.nl/sites/default/files/2018-11/Reference-Manual-Bevi-Risk-Assessments-version-3-2.pdf

