- 28 Oct 2024
- 4 Minutes to read
Fire triangle and tetrahedron
- Updated on 28 Oct 2024
- 4 Minutes to read
What is a fire and how can it arise?
Fire is a chemical reaction characterised by the rapid combination of a substance with an oxidant, typically oxygen, resulting in the release of energy in the form of heat, smoke, flame or any combination of these.
Fire Triangle
A fire can arise when the 3 components of the Fire Triangle - heat, fuel, and oxygen - are present simultaneously.
.png)
Fire triangle
Removing any of these elements can prevent or extinguish a fire. Various fire extinguisher types and fire suppression methods are designed to eliminate these elements to effectively combat fires:
Water extinguishers cool the fire, thus, removing the heat element from the Fire Triangle.
Carbon dioxide extinguishers and fire blankets work by displacing or removing the oxygen element from the Fire Triangle.
Flame-retardant materials act on the fuel element by slowing down or inhibiting the combustion process.
In addition to these fire suppression methods, proper design and adherence to process safety protocols are essential for eliminating ignition sources and preventing fires (Bosch, 2005).
Fire Tetrahedron
The Fire Tetrahedron is an expanded model that incorporates a fourth element, the chemical reaction, into the traditional Fire Triangle. Just like the Fire Triangle, the removal of any one of these elements results in the extinguishment of the fire. This understanding provides a foundation for us to delve into the explanation of various aspects of fire safety (Perry, 1997).
.png)
Fire Tetrahedron
Components of a Fire Tetrahedron
Fuels
Any material that can undergo combustion is considered a fuel. Fuels can be classified into solids, liquids, or gases, each with unique properties and behaviours during combustion. This classification system provides a valuable framework for understanding the distinct characteristics and behaviours of different fuel types, crucial information in the realm of fire safety and combustion. Before ignition, fuels may undergo diverse chemical or state changes before becoming active participants in a fire.
Gases
Gaseous fuels, such as natural gas and propane, are highly flammable in their vapour form. Their combustion is characterised by rapid ignition and high flame temperatures. The flammability of gases is characterised by flammability limits - concentrations in air within which they can ignite and sustain combustion.
Liquids
Liquid fuels include substances like gasoline, diesel, and various industrial chemicals. Liquids ignite more readily than solids and often produce flammable vapours that can form explosive mixtures with air. Flashpoint and autoignition temperature are critical characteristics influencing the flammability of liquid fuels. Prior to the ignition, liquids can evaporate while retaining their chemical composition or undergo decomposition and subsequent evaporation.
Solids
Solids encompass a wide range of materials, including wood, paper, fabrics, and plastics. Different types of solids respond differently to heat. Some solids melt before forming fuel vapour, while others produce vapour directly upon heating. Some solids may undergo direct sublimation into gases or follow a path of decomposition (pyrolysis) before evolving into vapours, followed by their ignition. Other solids melt before forming fuel vapour, either maintaining their original chemical composition or decomposing, which adds another layer of complexity. The rate of combustion in solids depends on factors such as density, moisture content, and the presence of accelerants.
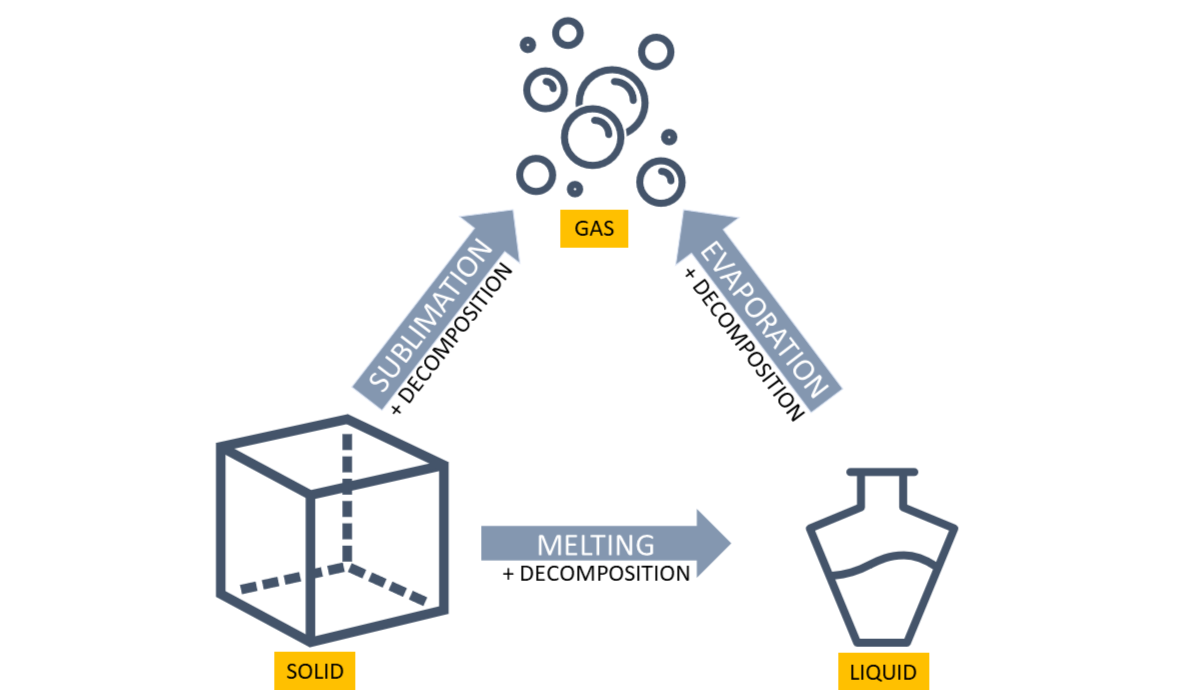
Phase transitions
Heat
The ignition of a flammable mixture can occur through various means. It may result from the flammable mixture encountering an external ignition source with sufficient energy or when the gas reaches an autoignition temperature, igniting without the need for an external source. The energy required for ignition depends on factors such as substance, concentration, pressure, and temperature. The table below presents the primary modes of ignition.
Piloted ignition | Auto-ignition | Spontaneous ignition |
|---|---|---|
Occurs when a flammable mixture encounters an external source such as a flame or spark, igniting the mixture. | Takes place when a flammable vapour mixture formed reaches its autoignition temperature, causing ignition without the presence of a flame, spark or other external ignition source. | Results from an exothermic chemical reaction leading to self-heating and ignition of the mixture without external ignition sources. |
Ignition sources are diverse, and challenging to fully eliminate entirely, including static electricity, hot surfaces, sparks, open flames, and electric circuits. Removing ignition sources has been recognised as an approach alone, but it is not entirely foolproof. Therefore, current fire prevention strategies focus on minimising ignition sources and preventing the formation of flammable mixtures.
Oxidising agent
Oxygen in the air is typically the oxidiser in the Fire Triangle, but other substances can act as oxidisers. Examples include:
Gases: fluorine and chlorine
Liquids: peroxides and chlorates
Solids: ammonium nitrate and certain metals
Additionally, exothermic decomposition, even without oxygen, is feasible, as seen with substances like ethylene oxide or acetylene.
Chemical chain reaction
The chemical chain reaction represents the self-sustaining nature of a fire once the ignition temperature of the fuel is reached. It involves the rapid combustion of fuel molecules in the presence of oxygen, leading to the release of heat and the generation of more flammable gases, which further perpetuates the reaction.
Conclusion
In conclusion, the transition from the Fire Triangle to the Fire Tetrahedron enhances our understanding of fire dynamics by emphasising the synergy of fuel, heat, oxygen, and chemical reactions. While the traditional model underscores the importance of these elements, the Tetrahedron introduces a crucial layer with the inclusion of a chemical chain reaction. This comprehensive model is invaluable for predicting and mitigating fire hazards. Exploring oxidising agents, ignition sources, and various fuel categories further highlights the diverse nature of fire scenarios, demanding tailored preventive measures. Analysis of fuel transformation pathways, from solid pyrolysis to liquid vaporisation and gaseous flammability limits, illuminates the intricate behaviours of materials in fire situations. This knowledge forms a solid foundation for robust fire safety protocols, emphasising prevention, intervention, and a nuanced understanding of different materials' reactions to fire.
References
Bosch, C. v. (2005). Methods for the calculation of physical effects 'Yellow book' CPR 14E. The Hague: Ministerie van Verkeer en Waterstaat.
Perry, R. H. (1997). Perry’s Chemical Engineers’ Handbook. McGraw-Hill: New York.

